J. Am. Chem. Soc. 2019, 141, 27, 10883-10904 full text link
文献汇报人:Hayatullah Imtiaz
汇报日期: 2019年12月14日
图文摘要:
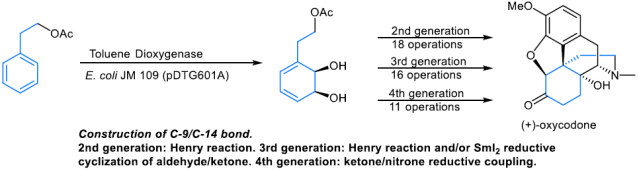
-
推荐原因:
Four distinct approaches to ent-oxycodone were designed and accomplished. All rely on the same starting material, the diene diol derived from phenethyl acetate by the whole-cell fermentation with E. coli JM109 (pDTG601A), a strain that overexpresses toluene dioxygenase.
The key step in the first-generation approach involves the construction of the C-9/C-14 bond by a SmI2-mediated cyclization of a keto aldehyde. The second-generation design relies on the use of the Henry reaction to accomplish this task. In both of these syntheses, Parker’s cyclization was employed to construct the D-ring. The third generation synthesis provides an improvement over the second in that the nitrogen atom at C-9 is introduced by azidation of the C-9/C-10 olefin, followed by reduction and lactam formation between the C-9 amine and the Fukuyama-type lactone. Finally, the fourth generation takes advantage of the keto−nitrone reductive coupling to generate the C-9/C-14 linkage. The four generations of the total syntheses of ent-oxycodone were accomplished in 13, 18, 16, and 11 operations (19, 23, 24, and 18 steps), respectively.
